Categories | Positive News
Article
Voluntary Recall Issued for Thyroid Medication Due to Sub-par Potency
October 2nd, 2020
•
Positive News
•
2 minute read
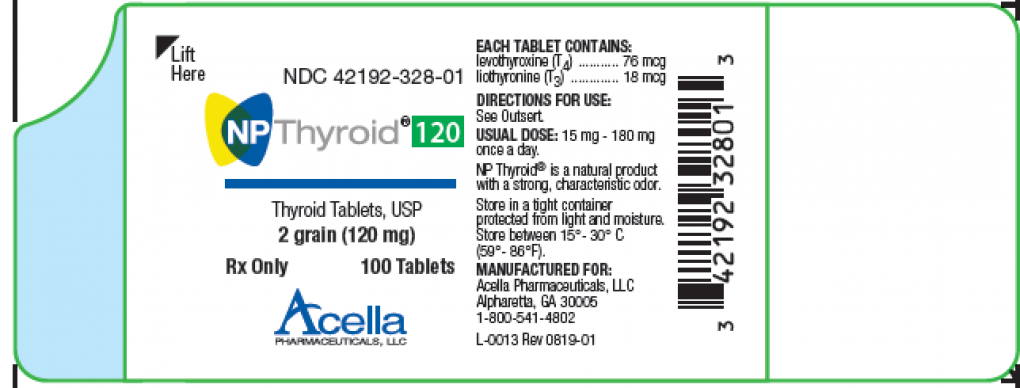
Voluntary Recall Issued for Thyroid Medication Due to Sub-par Potency
 Recalled Thyroid tablet; image courtesy of the FDA, www.fda.gov
Recalled Thyroid tablet; image courtesy of the FDA, www.fda.gov
Sources:
Thyroid Medications Recalled Because They May Not Be Strong EnoughAcella Pharmaceuticals, LLC Issues Voluntary Nationwide Recall of Two Lots of NP Thyroid®, Thyroid Tablets, USP Due to Sub Potency
About Brianna Smith
Brianna Smith is a freelance writer and editor in Southwest Michigan. A graduate of Grand Valley State University, Brianna has a passion for politics, social issues, education, science, and more. When she’s not writing, she enjoys the simple life with her husband, daughter, and son.
